This month, we’re focusing on the world’s greatest killer: cardiovascular disease. We were honored to speak with interventional cardiologist, cardiovascular researcher and educator Dr. C. Michael Gibson. Dr. Gibson has led groundbreaking work on heart attack pathophysiology, treatment and prevention and has transformed the way we approach cardiovascular disease. In our interview, he shares how we can alleviate cardiovascular disease to save lives and reduce global suffering. This conversation is vital, so don’t miss it.
A conversation with Dr. C. Michael Gibson
Despite countless therapeutic advancements over the past 50 years, a life is lost in the United States every 30 seconds due to cardiovascular disease. So what will it take to move the needle against this terrible health threat?
If anyone has an answer to this puzzling question, it’s Dr. C. Michael Gibson. He is the CEO of the combined non-profit Baim and PERFUSE clinical research institutes at Harvard Medical School as well as one of the most cited and respected heart health experts worldwide.
Dr. Gibson has led hundreds of clinical trials including some with over 30,000 participants. He helped discover what happens at the vascular level during a heart attack and established the related open artery and open microvasculature hypothesis. Dr. Gibson developed measures of coronary blood flow that are widely used today including the TIMI frame count and the TIMI myocardial perfusion grade. He is currently spearheading the Heartline™ Study using everyday technology like the Apple Watch to detect atrial fibrillation in people over 65.
According to Dr. Gibson, cardiology has changed dramatically during his career, but the field is still failing patients. To tackle the world’s biggest killer, he believes we need to “move upstream” to identify and prevent the disease before it strikes. Dr. Gibson argues that a proactive, not reactive, approach is necessary to prevent the large-scale devastation wrought by cardiovascular disease.

C. Michael Gibson, MD, Interventional Cardiologist, Beth Israel Lahey Health; CEO, Baim Institute for Clinical Research and PERFUSE; Professor of Medicine, Harvard Medical School
What inspired you to train as an interventional cardiologist?
As a child, I watched my grandfather struggle with heart failure. He survived, but he was one of the “walking wounded.” That was, in part, the impetus for me to become a doctor.

Dr. Gibson’s grandfather whose struggle with heart failure led to Dr. Gibson choosing a career in medicine
But an 18-year-old who landed in the emergency room influenced my decision to become a cardiologist. She was the first patient I saw as a medical student. She had been out all night partying, and she was in shock. We did an echocardiogram, which showed hypertrophic obstructive cardiomyopathy. She had an irregular and rapid heartbeat (known as atrial fibrillation or AFib). We shocked her heart back into normal sinus rhythm and gave her fluids, and she got better.
That night, before I presented the case in the morning, I read background material on the case written by Dr. Eugene Braunwald. I was fascinated by it. The physiology was mechanical and made sense to me. So when the program director asked where I wanted to go to residency, I said I wanted to work with Dr. Braunwald. He laughed and said: “He is the most powerful person in medicine. Not a chance!” Dr. Braunwald was the chairman of medicine at both Brigham and Women’s Hospital and the Mass General Brigham at the time.
But sure enough, a week later, I was on a plane to Boston. The rest is history. I’ve worked with Dr. Braunwald since 1986. His approach to diagnosis and treatment inspired me to work in interventional cardiology.
What has kept you focused on cardiovascular disease decades after your initial training?
My grandfather had a heart attack at age 45. All they had for him was some oxygen and morphine and even some cigarettes sold down the hallway. Fast forward to now.
Cardiology is an amazing field. It’s very evidence based. We conduct trials enrolling tens of thousands of patients to give definitive answers to important questions. I’m proud to have led many of those mega trials over the years.
I’ve seen the introduction of aspirin, anticoagulants, thrombolytics, lipid-lowering drugs, antiplatelets and a host of heart failure medicines. We’ve reduced mortality from 30% to about 6% in the setting of a heart attack. It has been unbelievably gratifying to be a part of that journey.
Beyond treatment, diagnosis has changed significantly. Previously, heart attack patients were waiting a long time to get treated. In the 1990s, I led the National Registry of Myocardial Infarction, where we tracked how long it took for heart attack patients to receive a drug or get an angioplasty. Nationally, we cut treatment time by about 30 minutes, which led to a 5% improvement in survival. It’s not just about giving the right drugs; it’s about making a timely diagnosis.
Why is heart disease so common and dangerous? What are the risk factors driving this chronic disease epidemic?
Often, we view heart disease as something that happens when you’re 60 years old, but it begins in childhood. We talk about secondary prevention—preventing another heart attack. That’s not enough. We talk about primary prevention— preventing the first heart attack in your 50s or 60s. That’s also insufficient.
“We need to focus on primordial prevention starting in childhood with diet, exercise and even medication for elevated cholesterol.”
That sounds scary to some people. But we now understand that someone’s “cholesterol years”—the amount of elevated cholesterol over time—is associated with risk. There are also certain inflammatory markers and very bad types of cholesterol that we didn’t recognize before. We need to treat people earlier for these factors.
How often do cardiovascular episodes recur?
About 800,000 heart attacks occur per year in the U.S. Almost a quarter of them are recurrent. If you have a recurrent heart attack, it doubles your risk of death and dramatically increases your risk of heart failure. You may live, but you’re among those walking wounded who have many symptoms and limited mobility and are likely to wind up back in the hospital.
What is the human toll, healthcare burden and economic cost associated with heart disease?

Dr. C. Michael Gibson with Dr. Eugene Braunwald, a pioneer in cardiology whose work expanded knowledge of heart disease in the area of congestive heart failure, coronary artery disease and valvular heart disease
What is the human toll, healthcare burden and economic cost associated with heart disease?
Heart disease is our biggest killer. It’s come close to becoming number two behind cancer, but strangely enough, we’ve seen a rise in age- adjusted mortality for heart disease in recent years. This rise stems, perhaps at least in part, from national guidelines removing specific targets for cholesterol reduction. We’re now returning to providing more specific guidelines.
Heart disease is not only a major killer, but living with the disease as the walking wounded is very expensive. Obviously, patients with heart failure or bleeding from their medications get costly procedures like stents and hospitalizations. Patients also often experience strokes. If you have a disabling stroke, your care will cost hundreds of thousands of dollars per year. We grossly underestimate the cost of vascular disease.
We also don’t always account for the related loss of economic contribution to society. With heart disease and certain types of Alzheimer’s and dementia that are vascular in origin, previously economically productive people become homebound or bedbound, and they’re not contributing.
Meanwhile, their caretakers may have to take time off work to care for them or drive them to their doctor visits. This carries a huge burden that is difficult to even capture.
How do the rates of heart disease and vascular disease vary across the globe?
The rates and types of vascular disease vary dramatically. Japan has much higher rates of bleeding stroke than the United States, for example. Some differences are driven by genetic, nutritional or environmental factors.
We have exported highly processed and fast food to some countries, such as China and India and their risk of heart disease is now going up. Sadly, they’re getting away from their native diets and adopting an unhealthy Western one.
What are the top three scientific or public health advancements in the field? And what progress has resulted?
Prevention, aborting heart attacks and treating the walking wounded have been our biggest advances. Regarding prevention, statin therapy emerged to reduce cholesterol in a cheap, cost-effective way. In some countries, you can now get statins over the counter. We can reduce people’s risk with those medications and should be using them earlier.
The second big advance was in heart attack care. If you’re having a heart attack, we can now open up your artery and stop it. That reduced mortality significantly.
The third advance was in heart failure care. We’ve introduced a succession of medicines that each attack a different problem with the pumping of the heart. Together these advances led to improvements in mortality and morbidity.
What major breakthrough technologies have we seen in cardiology over the last decade? What do you expect to see in the next 10 years?
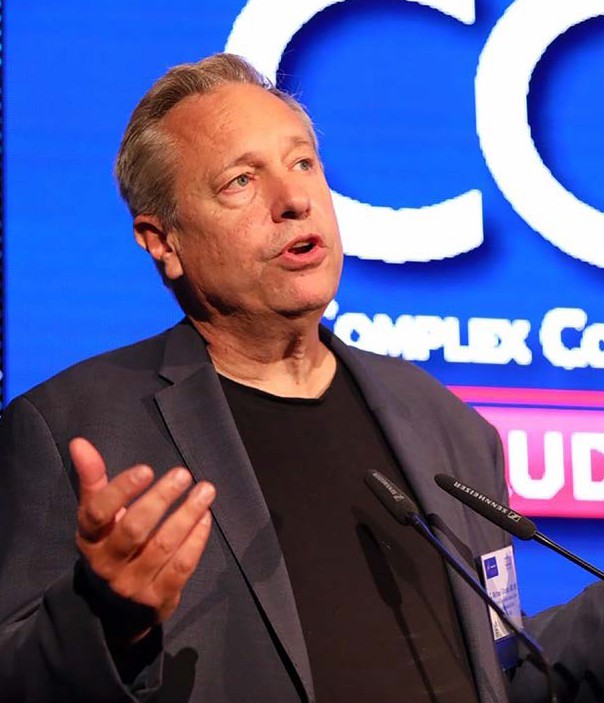
 Dr. Gibson speaking at an event hosted by the American Heart Association. He has led hundreds of clinical trials and is currently spearheading the Heartline™ Study
Dr. Gibson speaking at an event hosted by the American Heart Association. He has led hundreds of clinical trials and is currently spearheading the Heartline™ Study
Wearables and implantables—devices that monitor health, such as Apple Watches or Fitbits—have exploded. Many people wear them, and some are concerned that we are creating the “worried well.” There may be an element of that, but the larger benefit is that we are diagnosing more diseases that we didn’t recognize in the past.
One example is atrial fibrillation (AFib). Almost 33% of strokes have AFib as a cause, and people who have AFib are five times more likely to have a stroke. Yet up to 30% of people living with AFib aren’t diagnosed because the arrhythmia is often symptom-free. My colleagues and I are exploring the use of wearable technologies, such as the Apple Watch in the Heartline™ Study, to detect AFib earlier.
Previously, medicine focused on treating people after they become sick or symptomatic. Now, we’re targeting people who don’t currently have the disease and detecting it when it begins. Rather than bringing the patient to the trial, we’re bringing the trial to the patient.
We can’t afford to spend up to $4 billion testing a new drug or device. So the Heartline trial is a novel virtual trial with no brick-and-mortar lab or facility. We randomized over 32,000 people to use an Apple Watch via mail order or the Heartline app alone on their iPhones. They didn’t have to see a doctor or nurse and consented virtually. Whether a participant is hospitalized with a cardiac event is collected through the insurance claims database.
About 95% of people entered detailed information about how they were doing or feeling into the Heartline app. They shared a range of factors related to their health: weight; dietary, exercise and sleep habits and anxiety and depression levels. We now have terabytes of information stored securely to interrogate the relationships between these health measures and other measures of heart health. So it’s a really exciting time to be in cardiology research to glean these insights.
How might this novel format for clinical trials change the way we conduct science in the future?
We learned a lot trying to enroll people in the study, which was quite challenging during the COVID-19 pandemic. We had to aggressively seek out participants. We had 380 million impressions on social media, television, print and radio. We are going to publish that strategy as a road map for others.
It’s not just about finding participants but keeping them engaged. People don’t want to answer a few questions now and then. They want a wellness program that goes beyond their heart health. We are awash in disinformation. People are relying on trusted healthcare providers more than ever to sort through what’s out there and give a balanced view. So we provide wellness news that participants can consult daily for credible information, too.
How is artificial intelligence (AI) reshaping the vascular disease space?
We are at a tipping point. We are seeing amazing advances in imaging, diagnosis and EKG interpretation. For instance, the Mayo Clinic is determining if a patient has an enlarged heart or may go into heart failure via AI interpretation of an EKG.
Soon we’ll see AI powering diagnosis and guiding treatment more. In rural settings, AI can help predict that someone has heart failure via a smartphone connected to a small echo probe. Any allied healthcare professional can use the probe to capture a perfect picture of the heart. Within 30 seconds, an AI algorithm analyzes everything and spits out quantitative measurements of the heart.
We have the tools we need. Now we’ve got to figure out how to harness them. You can have great basic science, but it’s all for naught if you don’t have implementation science.
I’m optimistic about AI, but we need to be suitably skeptical to ensure that it’s used rigorously. There are always risks. You have to take a look inside the “black box” and make sure that it’s doing what you think and offering accurate, independent prognostic information.
How is the Guardian system from Avertix improving and saving lives via cardiac monitoring and alerting?
Back in 2001, I penned an article called “Time Is Muscle.” It was written to encourage people to call 911 if they think they’re having a heart attack. The article ended up being published on the same day as the September 11 attacks. So the information campaign was overshadowed by those events and stumbled at the beginning.
After publication, I received a call from an inventor who said he had a way to alert people effectively. This was 22 years ago. At that time, I was also working on the National Registry of Myocardial Infarction with the goal of getting people to the catheterization lab quicker when they got to the hospital. The problem was that people were coming in two and a half hours after their first chest pain. Decades later, we’re not doing any better.
“To improve heart attack care, we need to get people to the hospital faster.”
If we can shave off two and a half hours, we can shave off 2.5% in mortality. Alarming is one strategy. We worked on the Guardian implantable device for over two decades. If there’s a sign of a heart attack, the Guardian alarms and prompts people to get to the hospital quicker. In a study that led to the device being approved by the FDA, we also detected over 40 silent heart attacks in about 1,000 patients. These patients had no symptoms, but the alarm went off, and they were treated. Not all heart attacks cause someone to bend over with chest pain.
The Guardian is more accurate than symptoms alone, can detect silent heart attacks, get you to the hospital quicker and perhaps improve outcomes. Skeptics feared that the device would flood the healthcare system with needless cases. But we found that the positive predictive value of chest pain symptoms was better with the alarm on.
I have been critically ill myself with acute respiratory distress syndrome in an intensive care unit (ICU). The ICU is a very scary, complex setting. It can be a very traumatic experience. People who have a heart attack or spend time in a coronary care unit often experience a form of post-traumatic stress disorder.
Patients using the Guardian report an improved sense of well-being and say: “I felt much more relaxed. I felt as though I was bringing the intensive care unit home with me, like someone was there watching over me.”
Despite these advancements, deaths from heart disease are still alarmingly high. What will it take to bring rates down significantly?
We are on the precipice of an insane new world. There are forthcoming gene editing therapies designed to lower cholesterol. If you have cardiac amyloidosis (like Alzheimer’s of the heart), where scar tissue infiltrates the heart, you can reduce the disease by 95% with gene editing. In mice, we can now genetically edit hypertrophic obstructive cardiomyopathy.
“To drive rates down, we need to prevent the disease in the first place.”
We are going to see a major focus on early lifelong treatment and primordial prevention of these diseases with gene editing. There will be a broader early diagnosis of diseases using AI, blood tests and imaging studies. We’ll continue to see the introduction of new classes of effective medicines, too.
What do you personally do to keep yourself healthy?
I have always been concerned about getting heart disease myself. I eat a healthy diet and keep my weight down. My blood pressure is typically 100/60; my resting pulse is 60. I thought I was in great shape. But I got a calcium scan of my heart, and my score was very high, meaning I had calcification in my coronary arteries. Even though my LDL cholesterol was relatively low, another bad cholesterol called lipoprotein(a) or Lp(a) was markedly elevated. We’ve learned that it’s not just about managing the regular cholesterol types that we’re familiar with. There are particularly dangerous types like Lp(a) to monitor.
About 20% of Americans have elevated Lp(a), which puts them at a much higher risk of heart disease. But most don’t know they have it, because the test isn’t widely used. People need to ask for the test. We’ve only scratched the tip of the iceberg in terms of screening.
But the sad and scary thing is that lifestyle changes don’t reduce your Lp(a). You can’t stop eating Lp(a) or exercise it away. It’s just something your liver makes. You need medicine to turn it down. In some cases, a PCSK9 inhibitor may help. There are two drugs being studied right now that are small, inhibiting molecules of RNA that prevent the manufacture of Lp(a). I and others like me are waiting for these trials to conclude so we might access this medicine.
What should people do to lower the risk of heart disease on a day-to-day basis?
“Heart disease is a lifetime disease. Keep moving throughout your life. Know your numbers. Know your risk.”
Dr. Gibson is widely recognized for the research leading to the understanding of what happens at the vascular level during an acute myocardial infarction as well as the open artery and open microvasculature hypothesis in the setting of a heart attack
Check in with your doctor about your heart health.
It’s pretty simple: exercise as much as you can. It doesn’t have to be every day. You can pack it in on the weekends and get the same benefits.
In terms of nutrition, the food pyramid needs to be reinvented. Processed foods, cereals and sugars aren’t good for you. Eat mostly vegetables, fruits and protein. That’s the best diet for most people. Less is more, quantity-wise.
The most staggering advance in cardiovascular disease will be the treatment of obesity. These new GLP-1 agonists are incredible. In initial studies, these drugs can reduce someone’s weight by 15%. The mechanism by which these medications work is multifactorial: They curb cravings and make you less hungry. Then when you do eat, you feel fuller sooner. I personally lost 15% of my body weight using them. It was absolutely amazing.
There are triple agonists coming along that can reduce your weight by 24%. These medications will be revolutionary in terms of atherosclerosis and its downstream effects. So far, we have not seen any untoward effects. When people use these drugs, everything moves in the right direction: Blood pressure, LDL and hemoglobin A1C go down, and HDL goes up.
We need to stop treating obesity as a moral failing. It’s not. It’s a complex, multifaceted hormonal disease exacerbated by the culture that we live in. We need to treat it like other diseases. We should be reducing obesity through primordial prevention in children, sometimes with medication.
What will it take to bring your vision to life? Is there currently enough funding or attention to move the needle?
It’s complex. We need adequate funding for innovation to develop new compounds and strategies. But we can’t afford to pay for those forever. There are some efforts to curtail the duration of exclusivity, down from 15 to 20 years to closer to 8 to 10 years, to allow drugs to become generic sooner.
We need people who will innovate and get paid to develop new drugs initially. But at a certain point, we need to make those drugs affordable. Who’s going to manufacture affordable drugs if the profit margins aren’t there? This may be an anathema to some, but we need to consider whether countries or governments should manufacture generic drugs so that everyone can use them. If the business model isn’t supporting affordability, we need to rework our healthcare system and industries to make these drugs accessible.
People complain that almost 18% of our economy is dominated by health care. Maybe that’s a good thing. There will be many jobs taken away by AI and emerging technologies. But we all want to be alive and out of the hospital. Rather than having people unemployed, maybe we should focus their efforts on caring for each other and improving public health. Let AI make cars or computers.
What is your ultimate goal?
My ultimate goal is to put myself out of business as an interventional cardiologist. I want us to move upstream, prevent and identify the disease earlier in life. We currently have the ability to do this cheaply and rapidly. That’s the path forward.
This interview has been edited for length and clarity.
If you have any questions or feedback, please contact: curalink@thecurafoundation.com
Newsletter created by health and science reporter and consulting producer for the Cura Foundation, Ali Pattillo, consulting editor, Catherine Tone and associate director at the Cura Foundation, Svetlana Izrailova.